Abstract
Introduction. Despite the recent approval of several drugs for the treatment of AML, the 3 + 7 regimens remain as the standard of care for many patients. Its lack of efficacy represents the main cause of death, since only 10% of patients who show refractoriness/relapse overcome the disease. Therefore, there is still an urgent need for seeking more effective treatments. Aberrant RNA splicing has been described in AML, but its relevance as mechanism of resistance is unclear. In this study, we deepen the mechanism of resistance to cytarabine and the role of splicing factors SR proteins, involved in the spliceosome functionality, to seek more effective therapies for AML.
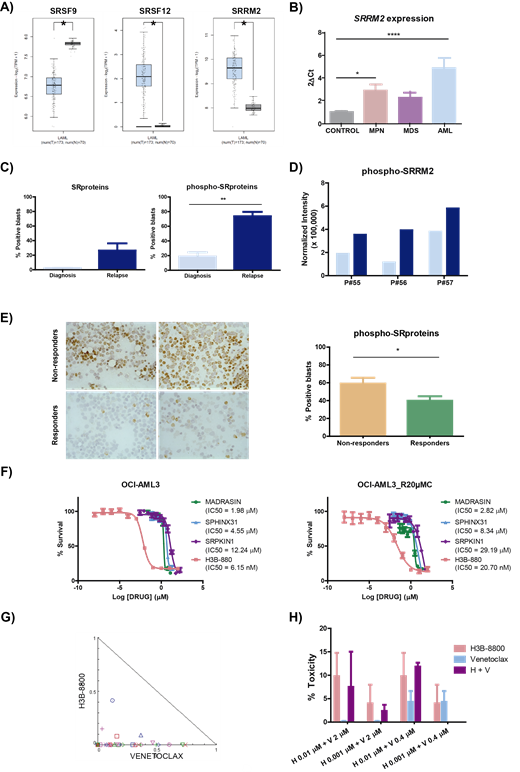
Methods. First, the expression levels of genes encoding SR proteins were analyzed with the GEPIA2 platform, comparing the data from the TCGA-LAML (AML patients) and GTEx (healthy) projects. Then, the gene expression of one of the most overexpressed genes, SRRM2, was validated by qPCR in samples of AML patients compared to controls and other myeloid disorders, as MDS and MPN (n=54). The resistance-associated phospho-proteomic profile was analyzed by LC-MS / MS after IMAC enrichment in paired samples from 3 AML patients. The expression of SR proteins and their phosphorylated forms was studied by immunohistochemistry (IHC) before and after resistance in paired bone marrow samples from 3 AML patients. We also analyzed by IHC the prognostic value of phospho-SR proteins at the moment of diagnosis in 64 patients with different responses to cytarabine (non-responders and responders). In order to validate an altered function of SR proteins, the analysis of the differential use of exons of paired samples from 25 AML patients was performed using RNAseq. Then, we evaluated in vitro the efficacy of some splicing modulators, and its combination with other approved drugs, in cytarabine-sensitive and resistant cells. The combination of H3B-8800, a spliceosome inhibitor, with venetoclax was tested in ex vivo samples from AML patients and healthy donors.
Results. We found that the gene expression levels of SRSF9, SRSF12 and SRRM2 were altered in AML (Fig 1A-B). Immunohistochemical studies revealed that, although at the protein level no differences were found in SR proteins expression between the diagnosis and relapse moment, an increase in the levels of phosphorylated SR proteins was associated at the time of relapse (Fig 1C). Indeed, the phosphorylation levels of SRRM2, among other SR proteins, were found to be increased during cytarabine resistance by phospho-proteomics (Fig 1D). Moreover, the phosphorylation levels of SR proteins predicted the response to cytarabine treatment, as AML patients that were non-responders presented significantly higher levels compared to responders ones (Fig 1E). The observed alterations in the phosphorylation of these proteins were correlated with a differential use of exons in some of their known targets, when comparing the diagnostic condition and drug resistance moment. Based on this evidence, the efficacy of combining different therapeutic options was evaluated in vitro using sensitive or cytarabine-resistant cell models (Fig 1F). The combination of H3B-8800 together with venetoclax was the most effective in vitro and also presented synergic effects ex vivo in AML patients samples (Fig 1G). Furthermore, this combination did not show toxicity over healthy hematopoietic progenitors, since the same doses that were effective in AML did not show toxicity in a healthy context (Fig 1H).
Conclusions. The results of this work shed light on the role of the RNA splicing process in cytarabine resistance in AML. Interestingly, the high levels of phosphorylated splicing factors SR proteins at diagnosis in refractory patients, would allow us to use them as a predictive biomarker of response to cytarabine treatment. Otherwise, due to the need to search effective and safe treatments in this disease, we have found that the combination of splicing inhibitors with venetoclax should be a good strategy for the treatment of AML.
Acknowledgment. This work has been possible thanks to the granting of the project PI19/01518 from the Carlos III Health Institute and the CRIS Against Cancer Foundation. ML.M. enjoys a research grant from the Spanish Society of Hematology and Hemotherapy and R.GV. a FPU grant from the Ministry of Science, Innovation and Universities.
Sanchez: Altum sequencing: Current Employment. Ayala: Incyte Corporation: Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Astellas: Honoraria; Celgene: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal